Surpass (1% Diclofenac Sodium) Topical Cream for Horses, 124 g, 1 Tube
By Surpass
Prescription Required
Have a Chewy account?
Sign in to quickly add pet health details.
$92.69 Autoship Price
Never miss a dose with easy refills
Skip, change, or cancel anytime
$92.69 Chewy Price
($92.69 /ea)
Save $7.40 (-7%)
$100.09 List Price
Tooltip Available
Promo, Save 20% on first Rx order with code RX20
Promo, New Customers Only: Spend $49+, Get $20 eGift Card + Free Shipping with code: WELCOME
In Stock
FREE delivery
Usually arrives in 3-5 days
Prescription approval made easy
We’ll reach out to your vet clinic.
Tooltip Available
Medications ship separately. Delivery times may vary.
Tooltip Available
Frequently bought together
- Kensington Protective Products Signature Medium Weight Horse Turnout Blanket, 63-in, Atlantis$122.49 Chewy Price$174.99 List Price
- AniMed FSO Flaxseed Oil Blend Horse Supplement, 1-gal$34.99 Chewy Price$44.99 List Price$33.24 Autoship PricePromo, New Customers Only: Spend $49+, Get $20 eGift Card +1 deal
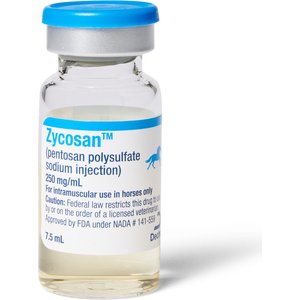
- Zycosan Injection for Horses, 250-mg/mL, 7.5 mL, 1 vial$115.50 Chewy Price$109.72 Autoship PricePromo, Save 20% On First Rx Order With Code: RX20 +2 deals
- Prescription Item
- Shires Equestrian Products ARMA Air Motion Brushing Horse Boots, Black, Full$39.99 Chewy Price$45.99 List Price
- Neogen Hypodermic 20 Gauge Needles, 1 Inch, 100 count$13.75 Chewy PricePromo, Save 20% On First Rx Order With Code: RX20 +1 deal
- Prescription Item
- Vetameg (flunixin meglumine) Paste for Horses, 30-g syringe$25.95 Chewy Price$24.65 Autoship PricePromo, Save 20% On First Rx Order With Code: RX20 +2 deals
- Prescription Item
- Durvet Chlorhexidine Solution Horse & Dog Antibacterial Wound Cleaner, 1-gal bottle$12.99 Chewy PricePromo, New Customers Only: Spend $49+, Get $20 eGift Card
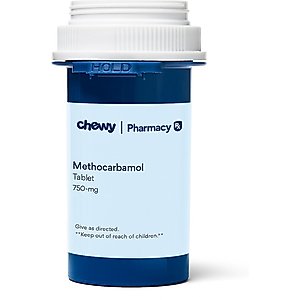
- Methocarbamol (Generic) Tablets for Dogs & Cats, 750-mg, 1 tablet$0.15 Chewy Price$0.14 Autoship PricePromo, Save 20% On First Rx Order With Code: RX20 +1 deal
- Prescription Item
- Adequan Equine i.m. (polysulfated glycosaminoglycan) Injectable for Horses, 100mg/mL, 50-mL vial$499.99 Chewy Price$474.99 Autoship PricePromo, Save 20% On First Rx Order With Code: RX20 +2 deals
- Prescription Item
- Bute (phenylbutazone) Phenylbutazone Powder for Horses, 1.1 lbs$38.99 Chewy Price$37.04 Autoship PricePromo, Save 20% On First Rx Order With Code: RX20 +2 deals
- Prescription Item